Surface tension heal thyself
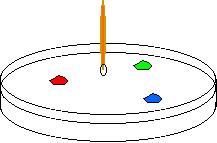
A petrii dish of milk wth 3 drops of food coloring is about to
be touched by a drop of soap on a toothpick.
Surface tension heal thyself

A petrii dish of milk wth 3 drops of food coloring is about to
be touched by a drop of soap on a toothpick.
Introduction
Soap will "break" or reduce the surface tension of water and milk. The non-soap surface tension will then pull open and thus enlarge, the patch of soapy surface. Drops of dye placed on the surface will move along with the surface.
Material
Optional
Optional
Assembly
Put a layer of milk about 1 cm (0.5") deep in the dish. Room temperature milk works better than cold milk.
To Do and Notice
Put one, two, or three drops of food coloring onto the surface of the milk.
Dip the toothpick into the dish soap so that there is a small drop of soap on the end of the toothpick.
Touch the surface of the milk with the soap drop on the end of the toothpick.
Notice that the food coloring streams away from the point where the soap touched the milk. After a while the motion of the food coloring will stop.
Sometimes the food color will stream all the way to the side of the dish and then reappear near the center.
Touch the milk with the soap drop on the toothpick again. Notice that the motion of the food coloring resumes.
What's Going On?
The milk is denser than water so the food coloring stays on and near the surface.
Soap reduces the surface tension of the liquid. The stronger surface tension of the surrounding liquid then pulls the surface away from the weak, soapy region. The food coloring moves with the surface, streaming away from the soap.
As the soap diffuses into the solution it surrounds fat globules in the milk, this is why fattier milks work better. As the soap is removed from the surface, surface tension returns to its original strength and the experiment can be repeated.
The surface will continue to stream away from a large drop of soap for a long time, until the soap has diffused into the solution and been taken up by the fat.
Eventually all of the fat globules in the milk will be surrounded by soap and some soap will remain on the surface causing surface-tension-driven convection to stop.
Going Further
Investigate using more or less soap, milk at different temperatures, different fat content milk: skim, 1%,2% half-and-half, or cream.
Raise the dish above a mirror by placing it on 3 film cans and notice that the food coloring appears on the bottom. Surface tension-driven-convection can produce motion of the food coloring from the top of the milk to the bottom and back to the top again.
Put a drop of strong alcohol into the milk. The alcohol will weaken the surface tension of the milk and cause the dye to move. The alcohol evaporates and also diffuses into the milk so that the surface tension returns to normal and another drop of alcohol can be added.
Etc.
Soap molecules have water-loving, hydrophilic, heads and water hating, hydrophobic, tails. The hydrophobic tails penetrate the fat globules of the milk forming a fat globule surrounded by soap called a micelle.
Credits
Modesto Tamez performed many experiments with surface tension driven convection that made this exploration possible.
|
Scientific Explorations with Paul Doherty |
|
9 January 2003 |